An innovative technology
- Axonics was established in late 2013 with the ambition to develop next-generation neuromodulation solutions for an improved patient and clinician experience
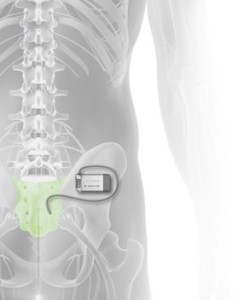
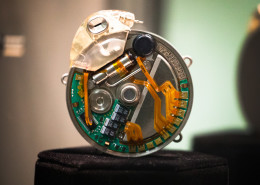
- The team is developing a complete system around a miniaturized rechargeable stimulator for the treatment of Overactive Bladder (OAB) and Fecal Incontinence (FI) as first applications
- It is protected by 19 issued US patents and EU counterparts as well as 10 new non-provisional patent applications filed
A large medical need
- Over 100 million people in the United States and Europe suffer from OAB and/or FI symptoms
- 3 million patients are not satisfied by conservative treatments or drugs and seek more advanced solutions such as Sacral Neuromodulation
A solid background
- Axonics is led by a team of seasoned medical device executives previously from Vessix Vascular, sold to Boston Scientific in 2012
- The technology is leveraging nearly 30 years of IP, technology and know-how building implantable devices from the Alfred Mann Foundation
- Timothy R. Deer, M.D., world renowned neuromodulation expert, is a co-founder and clinical advisor
- Axonics has a Clinical Advisory Board of 15 European and North American clinical experts in Sacral Neuromodulation
– See more at: http://www.axonicsmodulation.com/